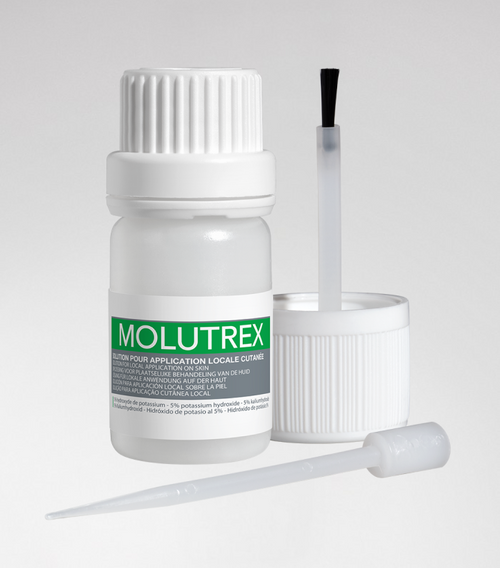
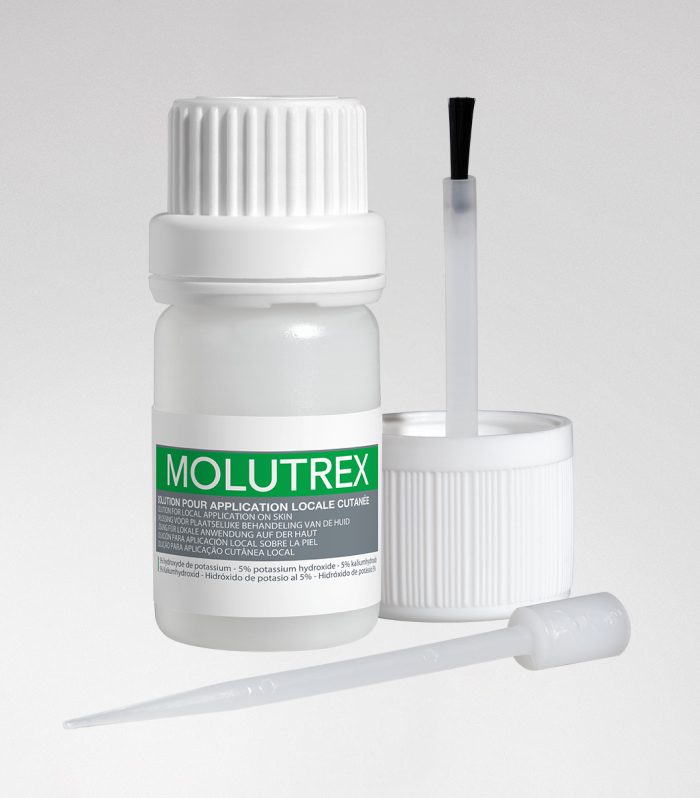
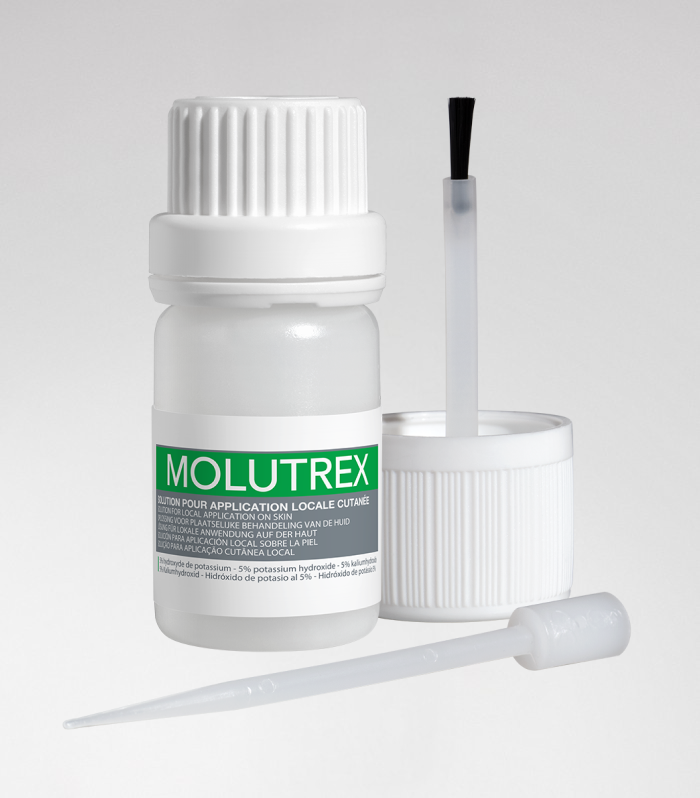
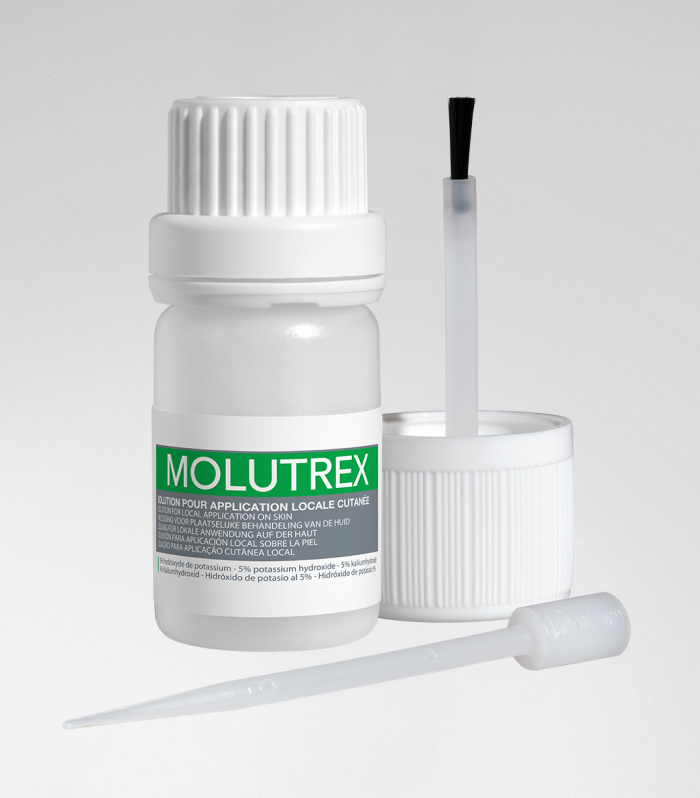
Molutrex
Molutrex
Sold out
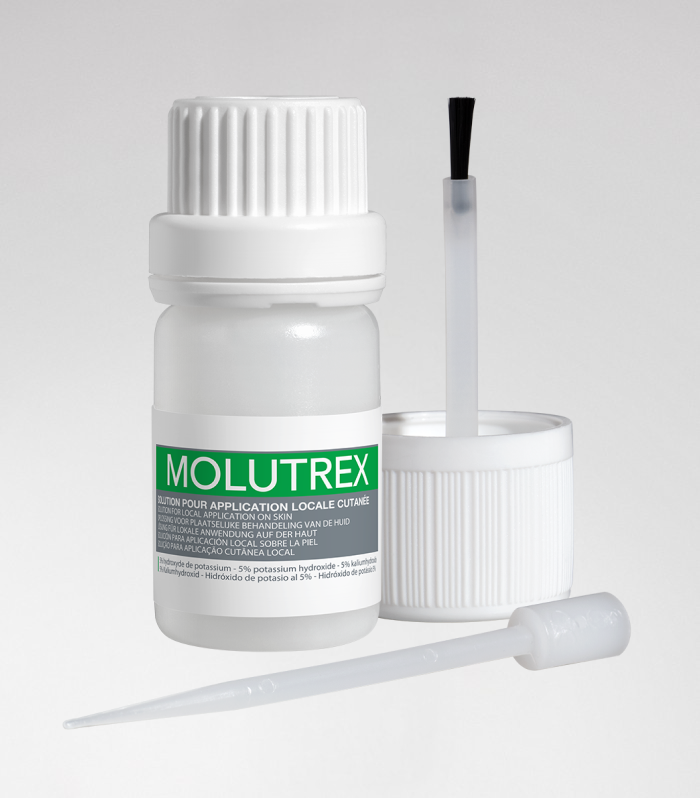




Molutrex - Molluscum contagiosum
Molutrex
5% potassium hydroxide solution for local cutaneous application.




BÉNÉFICES
INGRÉDIENTS ACTIFS
COMPOSITION
COMMENT L'UTILISER ?
FAQ
Where can I buy ACM Lab products?
Puis-je recevoir un catalogue de vos produits ?
Are the products tested on animals?
How long can I keep my product once opened?
How long can I keep my product before opening?